LAWSUITS NEWS & LEGAL INFORMATION
Premature Insulation Failure in Recalled St. Jude Medical Riata Defibrillator (ICD) Leads
Were you looking for Defective St Jude Cardiac Defibrillators Class Action lawsuits?
Washington, DC: In January, 2013, the FDA posted a statement concerning people with implanted St. Jude Riata or Riata ST Silicone Endocardial Defibrillation Leads. Riata and Riata ST leads connect an implantable cardioverter defibrillator (ICD) to the heart in order to monitor heart rhythms. ICD's can detect life-threatening heart rhythms and deliver an electrical shock from the ICD through the lead to the heart. ICD leads typically have layers of insulation that protect electrical conductor wires inside the lead.
Riata's manufacturer, St. Jude Medical Inc., recalled these leads on November 28, 2011, due to premature erosion of the insulation around the electrical conductor wires, known as insulation failure, or externalization. St. Jude Medical stopped selling these leads in late 2010 but more than 227,000 Riata leads had been distributed worldwide. According to St. Jude Medical, as of 2011, approximately 79,000 Riata leads remained implanted in patients in the United States.
Many factors contribute to the lifespan of an ICD lead, including the age and activity level of the patient. On average, an ICD lead is expected to last at least 10 years. The FDA is aware of an increase in frequency of reported Riata insulation failures, beginning approximately four years after implant. Insulation failure may cause some of the electrical conductors inside Riata leads to move within (migrate), or move entirely outside (externalize), the outer lead insulation. These changes may be detectable on X-ray or fluoroscopic imaging.
Lead insulation failure may cause the ICD lead to malfunction. ICD lead malfunction may cause abnormal sensing or pacing, or delivery of inappropriate or no shock therapy, which could result in serious adverse events, including death.
The FDA Safety Communication states there is currently not enough information to determine:
The recalled model numbers include the Riata (8F) Silicone Endocardial Defibrillation Leads (Models 1560, 1561, 1562, 1570, 1571, 1572, 1580, 1581, 1582, 1590, 1591, and 1592), and the Riata ST (7Fr) Silicone Endocardial Defibrillation Leads (Models 7000, 7001, 7002, 7010, 7011, 7040, 7041, and 7042).
In addition to the FDA Safety Communication for Riata and Riata ST Silicone Endocardial Defibrillation Leads, the agency also required St. Jude to conduct post-market surveillance studies on:
St. Jude Medical voluntarily recalled and stopped selling its QuickSite and QuickFlex LV CRT Leads on April 3, 2012. The FDA classified this as a Class II recall.
Riata lead injury lawsuits involving St. Jude Medical's Riata leads are currently being investigated. Numerous lawsuits involving St. Jude Riata leads have been filed in Minnesota (0:12-cv-01717-PJS-JSM) and California (8:13-cv-00383-JVS-AN) and the number is rising.
On January 9, 2014 Federal Judge James V. Selna denied St. Jude's Motion to Dismiss five cases alleging manufacturing defects causing their St. Jude Riata Leads to fail. The Honorable James V. Selna ruled in the case of In Re St. Jude Medical Device Litigation, SACV 13-383 JVS (AN) that Plaintiffs had met their burden of showing a plausible connection between the injuries they suffered and the alleged defective St. Jude leads under the Twombly/Iqbal pleading standard. Judge Selna also held that Plaintiffs stated claims parallel to federal requirements or premised upon violations of FDA regulations, and were therefore not preempted by federal law.
Last updated on
FREE RECALLED ST. JUDE MEDICAL RIATA DEFIBRILLATOR (ICD) LEADS LAWSUIT EVALUATION
Send your Recalled St. Jude Medical Riata Defibrillator (ICD) Leads claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
St Jude's Riata Claims
Many factors contribute to the lifespan of an ICD lead, including the age and activity level of the patient. On average, an ICD lead is expected to last at least 10 years. The FDA is aware of an increase in frequency of reported Riata insulation failures, beginning approximately four years after implant. Insulation failure may cause some of the electrical conductors inside Riata leads to move within (migrate), or move entirely outside (externalize), the outer lead insulation. These changes may be detectable on X-ray or fluoroscopic imaging.
ICD Lead Failure
The FDA Safety Communication states there is currently not enough information to determine:
- How frequently and how soon after implantation Riata insulation fails;
- How often and how soon both inner and outer layers of insulation fail and at what point migration or externalization of the electrical conductors cause ICD lead malfunction or other problems; and
- Risk factors that contribute to insulation failure or externalization of the electrical conductors.
Recalled St Jude ICD Leads
In addition to the FDA Safety Communication for Riata and Riata ST Silicone Endocardial Defibrillation Leads, the agency also required St. Jude to conduct post-market surveillance studies on:
- QuickFlex LV CRT leads
- QuickSite LV CRT leads
- Riata ST Optim and Durata ICD leads
St. Jude Medical voluntarily recalled and stopped selling its QuickSite and QuickFlex LV CRT Leads on April 3, 2012. The FDA classified this as a Class II recall.
St Jude Riata Lawsuit Update
On January 9, 2014 Federal Judge James V. Selna denied St. Jude's Motion to Dismiss five cases alleging manufacturing defects causing their St. Jude Riata Leads to fail. The Honorable James V. Selna ruled in the case of In Re St. Jude Medical Device Litigation, SACV 13-383 JVS (AN) that Plaintiffs had met their burden of showing a plausible connection between the injuries they suffered and the alleged defective St. Jude leads under the Twombly/Iqbal pleading standard. Judge Selna also held that Plaintiffs stated claims parallel to federal requirements or premised upon violations of FDA regulations, and were therefore not preempted by federal law.
Legal Help
If you or a loved one has suffered similar damages or injuries, please fill in our form and your complaint will be sent to a lawyer who may evaluate your claim at no cost or obligation.Last updated on
RECALLED ST. JUDE MEDICAL RIATA DEFIBRILLATOR (ICD) LEADS LEGAL ARTICLES AND INTERVIEWS
Faulty St. Jude Riata Defibrillator Leads Eyed for Link to Heart Infections
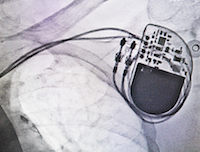
Washington, DC: Premature Insulation Failure in recalled St. Jude Medical Riata Defibrillator (ICD) Leads has been tentatively linked to polymicrobial endocarditis, a serious, often deadly condition that causes inflammation of the heart lining, muscles and valves. The decayed insulation on the electrical leads that connect the pacemaker to a patient’s heart may be a fertile breeding ground for bacteria and fungi that can attack a patient’s heart.
Patients who still carry recalled St. Jude Defibrillators leads in their chests have many reasons to be wary. This is another one that needs to be watched. Left untreated, endocarditis is always fatal.
Patients suffering from the condition may experience:
In previous studies, endocarditis has been linked to intravenous drug abuse, dental infections and surgical procedures. Having had heart surgery, including a pacemaker implant has long been recognized as increasing a patient’s risk for the condition. The infectious agent in most of the occurrences is streptococcal or staphylococcal bacteria or fungi, but for the most part, infections may be traced to a single source.
The studies of endocarditis in patients with premature insulation failure in recalled St. Jude Medical Riata defibrillator (ICD) leads shows something different. Polymicrobial endocarditis arises from several infectious agents.
There is, for some reason, more variety in the flora (referred to in the scientific literature as “vegetations”) involved in these cases. The findings suggest that, somewhere in the patient’s body, there is a fertile place for bacteria and fungi to flourish. The inquiry has moved to the possibility that the faulty insulation and resulting exposure (or externalization) of St. Jude Medical Riata defibrillator leads may be the host.
Thestudy that advances this theory notes that, “The externalization of Riata lead may cause the malfunction but it could also promote bacterial colonies and vegetations. In conclusion, looking for early signs of infection is mandatory during Riata leads follow-up checks.”
READ MORE
Insulation Failure, Battery Failure and Cyber Hacking - A Triple Threat for Pacemaker Patients

Washington, DC: Premature insulation failure in recalled St. Jude Medical Riata defibrillator (ICD) leads may be only the tip of the iceberg for heart patients with ICDs, more commonly known as “pacemakers.”
In 2011 St. Jude Medical Inc. recalled the electrical leads used to connect the Riata pacemaker to patients’ hearts because of flaws in the protective insulation. Failure of the insulation on an electrical lead can cause a pacemaker to deliver inappropriate stimulation to the user’s heart. For cardiac patients with abnormal heart rhythms, the disruption can be fatal. In addition, the exposure of the electrical lead has been linked to polymicrobial endocarditis, a serious bacterial infection of the heart . St Jude stopped marketing the defective leads in 2010, but thousands remain implanted in chests of patients in the United States.
The problems with St. Jude ICDs aren’t limited to the electrical leads. In 2016, the FDA issued a safety warning about the lithium batteries used to power them.
The ICDs are designed to alert patients to seek medical intervention to replace the battery approximately three months before it fails. St Jude has reported, however, that deposits of lithium, known as “lithium clusters,” can form within the battery, creating abnormal electrical connections that cause the battery to fail quickly. In some cases, full battery drainage has occurred in as a little as a day, leaving the patient with little or no time to get medical attention.
Finally, cybersecurity researchers at the University of Leuven in Belgium and the University of Birmingham in England have reported that they were able to successfully hack into ten different ICDs using relatively inexpensive, easily obtainable electronic components and a radio antenna.
In January 2017, the FDA warned that vulnerabilities in St. Jude Medical's ICDs might allow a hacker to deplete the battery or administer incorrect pacing or shocks. The hacker’s access point is a remote device programmer, intended to allow doctors and other medical professionals to adjust the settings on the pacemaker from up to five meters away.
St. Jude thereafter developed a patch to fix the devices’ susceptibility, but a later FDA warning letter suggests that the patch, itself, has not been adequately tested.
Patients who have an ICD manufactured by St. Jude Medical, Inc. would do well to seek medical advice about their risks and options.
READ MORE
Implantable Defibrillators with Faulty Batteries Lead to Class Action
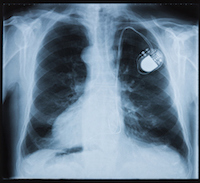

September 11, 2017
Washington, DC: Premature Insulation Failure in recalled St. Jude Medical Riata Defibrillator (ICD) Leads has been tentatively linked to polymicrobial endocarditis, a serious, often deadly condition that causes inflammation of the heart lining, muscles and valves. The decayed insulation on the electrical leads that connect the pacemaker to a patient’s heart may be a fertile breeding ground for bacteria and fungi that can attack a patient’s heart.
Patients who still carry recalled St. Jude Defibrillators leads in their chests have many reasons to be wary. This is another one that needs to be watched. Left untreated, endocarditis is always fatal.
Patients suffering from the condition may experience:
- High fever;
- New or different heart murmur;
- Fatigue;
- Muscle pain;
- Small spots from broken blood vessels under the nails, on the whites of the eyes, on the chest, in the roof of the mouth and inside the cheeks;
- Chest pain, shortness of breath, coughing;
- Night sweats;
- Headache;
- Blood in the urine; or
- Unexpected weight loss.
In previous studies, endocarditis has been linked to intravenous drug abuse, dental infections and surgical procedures. Having had heart surgery, including a pacemaker implant has long been recognized as increasing a patient’s risk for the condition. The infectious agent in most of the occurrences is streptococcal or staphylococcal bacteria or fungi, but for the most part, infections may be traced to a single source.
The studies of endocarditis in patients with premature insulation failure in recalled St. Jude Medical Riata defibrillator (ICD) leads shows something different. Polymicrobial endocarditis arises from several infectious agents.
There is, for some reason, more variety in the flora (referred to in the scientific literature as “vegetations”) involved in these cases. The findings suggest that, somewhere in the patient’s body, there is a fertile place for bacteria and fungi to flourish. The inquiry has moved to the possibility that the faulty insulation and resulting exposure (or externalization) of St. Jude Medical Riata defibrillator leads may be the host.
The
READ MORE
Insulation Failure, Battery Failure and Cyber Hacking - A Triple Threat for Pacemaker Patients

August 24, 2017
Washington, DC: Premature insulation failure in recalled St. Jude Medical Riata defibrillator (ICD) leads may be only the tip of the iceberg for heart patients with ICDs, more commonly known as “pacemakers.”
In 2011 St. Jude Medical Inc. recalled the electrical leads used to connect the Riata pacemaker to patients’ hearts because of flaws in the protective insulation. Failure of the insulation on an electrical lead can cause a pacemaker to deliver inappropriate stimulation to the user’s heart. For cardiac patients with abnormal heart rhythms, the disruption can be fatal. In addition, the exposure of the electrical lead has been linked to polymicrobial endocarditis, a serious
The problems with St. Jude ICDs aren’t limited to the electrical leads. In 2016, the FDA issued a safety warning about the lithium batteries used to power them.
The ICDs are designed to alert patients to seek medical intervention to replace the battery approximately three months before it fails. St Jude has reported, however, that deposits of lithium, known as “lithium clusters,” can form within the battery, creating abnormal electrical connections that cause the battery to fail quickly. In some cases, full battery drainage has occurred in as a little as a day, leaving the patient with little or no time to get medical attention.
Finally, cybersecurity researchers at the University of Leuven in Belgium and the University of Birmingham in England have reported that they were able to successfully hack into ten different ICDs using relatively inexpensive, easily obtainable electronic components and a radio antenna.
In January 2017, the FDA warned that vulnerabilities in St. Jude Medical's ICDs might allow a hacker to deplete the battery or administer incorrect pacing or shocks. The hacker’s access point is a remote device programmer, intended to allow doctors and other medical professionals to adjust the settings on the pacemaker from up to five meters away.
St. Jude thereafter developed a patch to fix the devices’ susceptibility, but a later FDA warning letter suggests that the patch, itself, has not been adequately tested.
Patients who have an ICD manufactured by St. Jude Medical, Inc. would do well to seek medical advice about their risks and options.
READ MORE
Implantable Defibrillators with Faulty Batteries Lead to Class Action

August 12, 2017
Toronto, Ontario Thousands of Canadians and American cardiac arrhythmia patients with implantable defibrillators powered by faulty ion batteries and manufactured by St. Jude’s Medical Inc. and St. Jude’s Medical Canada are or will soon be eligible to join class actions suits in Canada and the US. READ MORE
READ MORE St Jude Heart Valve Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News

READER COMMENTS
Dale May
on
I feel that the Riata leads that were placed into my heart and then subsequently removed because they were defective have caused medical complications that have severely changed my life for the worse. I want St. Jude to be held responsible for this horrible affect on my life. I spend every day worrying about having another arrhythmia that may cause additional heart muscle damage and possibly kill me.
William Alexander
on