| Company: | ISIS Pharmaceuticals, Inc. |
| Ticker Symbol: | ISIS |
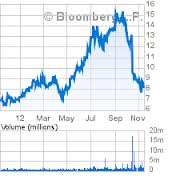
| Class Period: | Mar-29-12 to Oct-15-12 |
| Date Filed: | Jan-7-13 |
| Lead Plaintiff Deadline: | Mar-8-13 |
| Court: | Southern District of California |
| Allegations: | |
The Complaint alleges that the Company conditioned investors to believe that Kynamro (mipomersen sodium), one of the drugs in its pipeline, would receive approval from the U.S. Food and Drug Administration ("FDA") through a host of materially false and misleading statements regarding the safety and efficacy of the drug, as well as reportedly positive results from Kynamro's phase three clinical trial. As a result of the foregoing, the Company's statements were materially false and misleading at all relevant times.
On October 16, 2012, two days prior to a meeting of the FDA'sEndocrinologic and Metabolic Drugs Advisory Committee to decide on whether to recommend Kynamro for FDA approval, the FDA published a clinical briefing document questioning the safety and efficacy of Kynamro. Specifically, the report noted that abnormal growths or neoplasms developed in 3.1% of patients treated with Kynamro, as compared to only 0.9% of patients who took a placebo. The FDA report concluded that this "imbalance in neoplasms will need to be assessed further in on-going and future studies and post-marketing (if approved)." In addition, the report found that three patients treated with Kynamro died during clinical testing. Two died of heart attacks and the other patient died of acute liver failure, and the report concluded that "the potential for a contributing effect of mipomersen cannot be ruled out."
If you acquired the securities of the defendants during the Class Period you may, no later than the Lead Plaintiff Deadline shown above, request that the Court appoint you as lead plaintiff through counsel of your choice. You may also choose to remain an absent class member. A lead plaintiff must meet certain requirements.