 Top Class Action Lawsuits
Top Class Action Lawsuits
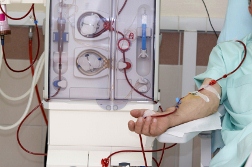
Dialysis Death Lawsuit Update. The lawsuits continue against Fresenius Medical Care, the maker of Granuflo and Naturalyte. This week, they found themselves facing a wrongful death class action lawsuit filed by the widow of Earin Blossom. The potential class action, filed in the Northern District of California, alleges the makers of Granuflo and Naturalyte, and their subsidiaries “failed to exercise reasonable care in manufacturing and selling defective dialysis products known as Granuflo and and Naturalyte.”
Tina Nunn, who filed the lawsuit on behalf of herself, her late husband, and those similarly situated, alleges that the dialysates caused fatal complications and sudden death, and caused her husband to incur substantial medical expenses prior to his death.
According to court documents, Earin Blossom began hemodialysis treatments in November 2010. During the course of those treatments, which took place three times a week at a Fresenius dialysis clinic in Fremont, he received both Granuflo and Naturalyte additives. Then, on April 6, 2011, just a few hours after completing a dialysis treatment at the clinic, Blossom suffered a massive heart attack and died.
The lawsuit alleges that Blossom’s metabolic alkalosis, cardiac arrest and subsequent demise were a direct and proximate result of his use of Granuflo and/or Naturalyte. The lawsuit also claims that the defendant knew its products resulted in excess bicarbonate levels in patients, often leading to metabolic alkalosis, a dangerous condition associated with heightened risks for heart attack, cardiac arrhythmia and sudden death.
Both Fresenius Medical Care products—Naturalyte and GranuFlo—are used in the treatment of acute and chronic renal failure during hemodialysis. The concentrate is formulated to be used with a three-stream hemodialysis machine, which is calibrated for acid and bicarbonate concentrates, according to the FDA safety recall initiated in March 2012. The recalled Naturalyte Liquid Acid Concentrate and Naturalyte GranuFlo (powder) Acid Concentrate was manufactured and distributed from January 2008 through June 2012.
An internal memo issued by Fresenius on November 4, 2011 warns that the GranuFlo and NaturaLyte products could lead to a greater risk of cardiac arrest and other heart problems. The memo, which was anonymously leaked to the FDA earlier this year, warned doctors working in Fresenius dialysis centers only that 941 dialysis patients suffered cardiac arrest in 2010 from GranuFlo use. Dangerously high biocarbonate levels would put their patients at a risk of cardiac arrest up to six times higher than that of patients using competing products.
No comment.
Top Settlements
Auto Workers Get $6M. And justice for all…all employees that is—and in this particular case it takes the form a $6 million settlement of a California labor law class action lawsuit alleging discrimination and unfair dismissal. Brought by former employees of Freemont-based New United Motors Manufacturing, Inc. (NUMMI), California’s last auto plant, the lawsuit, alleged that employees who were on disability at the time of the plant closure, were denied the severance benefits.
Specifically, the NUMMI workers’ lawsuit alleged that employees who were on disability in the period between October and the plant’s closure on April 1st did not receive benefits and services offered to employees who were not on disability during that same period. Those benefits and services included a severance package including a base payout with an additional retention bonus determined by years worked at the plant. The plant employees working between October 1st and April 1st were also offered transitional services, including access to a one-stop center, career and educational fairs, and skills assessments.
The plaintiffs also claimed that they, being on leave due to their disabilities and/or NUMMI’s refusal to accommodate their disabilities, were unjustly denied the bonus enhancement and transitional services. Further, the workers alleged in their complaint to the Equal Employment Opportunity Commission (EEOC) that they were physically capable of returning to work during the severance period, but were denied reinstatement. The EEOC issued “right to sue” letters to several NUMMI workers, while retaining the right to continue its investigation.
While the EEOC charges were pending, a group of former employees filed a federal lawsuit in the United States District Court for the Northern District of California. The plaintiffs filed claims for declaratory and injunctive relief, as well as damages for violations of the Americans with Disabilities Act, The Fair Employment and Housing act, the Unfair Business Practices Act, and California’s Public Policies.
The plaintiffs sought reformation of the severance agreement, restitution, lost compensation and other employment benefits and compensatory and punitive damages, and reasonable attorneys’ fees and costs for the defendants’ violations of their rights. Defendants named in the suit included New United Motors Manufacturing, Toyota Motor Corporation and Toyota Motor Sales. A class certification was later granted. Prior to the EEOC’s filing its own lawsuit, the matter was resolved via settlement for $6 million. As a part of the settlement, NUMMI entered into a conciliation agreement with the EEOC.
Floxed But Not Fleeced? Last—but certainly not least—the 900lb gorilla—Bayer—reached a partial settlement in an antitrust class action lawsuit involving the prescription antibiotic Cipro. The lawsuit claims Bayer Corporation, Barr Laboratories, Inc., Hoechst Marion Roussel, Inc., Watson Pharmaceuticals, Inc., and The Rugby Group, Inc. violated antitrust and consumer protection laws by agreeing not to compete with each other, and by keeping lower-cost generic versions of Cipro off the market. This settlement is with Bayer Corporation only; the case against the other manufacturers continues.
FYI—neither the case nor the settlement is about the safety or effectiveness of Cipro. Bayer has paid $74 million into a settlement fund that will compensate consumers and third-party payors (Class Members) who paid or reimbursed for Cipro in California between January 8, 1997 and October 31, 2004. Cipro purchasers not eligible for settlement payments include: (1) anyone who received Cipro through the MediCal Prescription Drug Program, (2) anyone who purchased Cipro to resell it, (3) government entities, (4) the manufacturers and related entities being sued, and (5) all purchasers of Cipro who paid a flat co-payment and who would have paid the same co-payment for a generic version under their health insurance policy.
Individual payments will be based on the total number of valid claims filed and how much the Class Member paid or reimbursed for Cipro. Attorneys’ fees not to exceed one-third of the fund, litigation costs, and other fees and expenses will be deducted prior to distribution. Full details about the settlement can be found in the Settlement Agreement, which is available at www.CiproSettlement.com.
Class Members must submit a claim form by March 31, 2014 in order to get a payment. The claim form and instructions on how to submit, together with complete details of the settlement are available at www.CiproSettlement.com.
Ok Folks, That’s all for this week. Have a good one—see you at the bar!







 Top Class Actions
Top Class Actions Top Class Actions
Top Class Actions